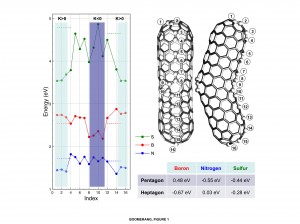
(Click image to enlarge.) Density functional theory calculations compare the energy necessary to substitute boron (red), nitrogen (blue) or sulfur (green) atoms for carbon atoms at 16 positions on a curved carbon nanotube (right). Where the curve is negative (K < 0), the carbon rings have seven atoms (heptagons) and it takes less energy for boron to substitute for carbon; boron selectively creates negative curves in the nanotubes and keeps the ends from closing, so they grow longer. Substitutional energy levels for nitrogen are lowest in pentagonal carbon atom arrangements, making nitrogen most likely to substitute for carbon at the positive (K > 0) positions. Researchers have found nitrogen gives rise to periodic raised rings along nanotubes, leading to bamboo-pole-like architectures. Sulfur can be accommodated in both pentagonal and heptagonal carbon rings and so promotes branched nanotubes.
In 2007, Bobby Sumpter and his Oak Ridge National Laboratory nanomaterials theory collaborators used computers to predict that adding a pinch of boron would produce kinks in carbon nanotubes. As an undergraduate student at Rensselaer Polytechnic Institute (RPI) the next year, Daniel Hashim guessed the curves might make these boron-doped nanotubes good energy-storing materials.
But no one expected what Hashim got when he actually used boron to contaminate the carbon in nanotubes – cylinders thousands of times thinner than a human hair.
“I didn’t know it was going to come out as a porous, three-dimensional solid framework” – a nanosponge big enough to hold in your hand, says Hashim, now a doctoral student at Rice University. “That was the big surprise. We didn’t have the insight at the time to understand that that’s what would happen.” Now researchers are touting these macro manifestations of nanotechnology as possible tools to clean oil spills, store energy, construct bones and meet other needs.
The Oak Ridge researchers used high-performance computing to help understand what’s happening at the atomic scale as these extraordinarily versatile black blocks grow. But it took an off-handed remark to link their work to the sponge-testing experimentalists. (See sidebar, “A spontaneous collaboration.” [1])
The scientists detail the material’s properties in a paper published online in Nature Scientific Reports:
• It repels water but sops up carbon-containing liquids, holding up to 100 times its weight in oil.
• The material is 99 percent air, so it’s remarkably light and absorbent.
• To get the oil out, just squeeze (tests show the sponges are still elastic after 10,000 compressions) or set on fire. The oil burns off, doing little harm to the reusable material.
• Because the material is made with an iron-based catalyst, magnetic fields can manipulate the sponges, making them easier to move or collect if they’re used for oil spill remediation or other purposes.
Boron, a common element that neighbors carbon on the periodic table and is found in laundry detergent, fiberglass insulation and other products, makes these high-tech sponges special. First, it “acts as a surfactant, which means it sits at the edge of the growth of carbon nanotubes and keeps them open,” says Sumpter, Chemical and Materials Sciences Group leader and director of Oak Ridge’s Nanomaterials Theory Institute. That helps the sponges grow to millimeters in length – extraordinarily large for such structures, says paper co-author Mauricio Terrones, a professor of physics, materials science and engineering at Pennsylvania State University and Japan’s Shinshu University.
More important, Sumpter adds, when boron is incorporated into the chicken-wire lattice of carbon atoms, it can induce an elbow – a slight bend – in the nanotube. Each nanotube could have multiple bends, the group’s calculations show, so “you get a network that winds through in a three-dimensional pattern.”
If that isn’t enough, at the elbows “the reactivity is different. If you have something close to it they’ll chemically bond across that,” so the nanotubes make sturdy connections. Things get messy, with kinked nanotubes bonding into Y-shaped and four-way junctions. This microscopic tube tangle grows into a remarkably sturdy macroscopic sponge.
Creating strong covalent chemical bonds – ones in which atoms share electrons – that weld carbon nanotubes has always been difficult, Terrones says. The secret, the researchers found, is introducing minute amounts of other elements, in this case boron, at temperatures as high as 850 Celsius.
Sumpter, Vincent Meunier (now an RPI professor) and their Oak Ridge colleagues used Jaguar, the Cray XT at the Oak Ridge Leadership Computing Facility (LCF), and other machines to computationally decode how boron creates nanotube defects. Their tool of choice is density functional theory (DFT), a quantum mechanical modeling method that calculates the arrangement and interactions of electrons in atoms and molecules.
“These are relatively large (atomic) systems” – multiple carbon structures, Sumpter says, each with odd defects that throw a wrench into the usual DFT calculations. Yet “it’s certainly doable these days, in particular with computing facilities” like the ORNL LCF.
In fact, for six years Sumpter and Meunier have calculated the topological defects induced by doping nanotubes with sulfur, nitrogen, phosphorus and other elements. “The strength of calculations is we often can do them much more efficiently” than experiments, Sumpter says. “We can ask a lot more questions about what happens, so we can probe through more elements than they can possibly probe experimentally in a reasonable amount of time.”
Using Jaguar, the researchers showed that boron atoms preferentially substitute for carbon atoms in a string along the inside of the bent tube, Terrones says. It was a surprising discovery, he adds, but researchers examined the sponges with high-tech instruments to confirm the theorists’ predictions.
Hashim was aware of the ORNL group’s early results in 2008, when he went to work with Terrones, then at Mexico’s Institute of Scientific Research and Technology, on a National Science Foundation (NSF) exchange. Hashim was studying with RPI materials scientist Pulickel Ajayan and thought boron impurities would make nanotubes better absorb lithium ions for battery applications.
To make the sponges, an aerosol of toluene (an industrial feedstock comprised of carbon and hydrogen), ferrocene (a compound of iron, carbon and hydrogen that catalyzes the reactions) and triethylborane (which provides the boron atoms) was released into a reactor chamber under high temperature.
While in Mexico, Hashim gradually tweaked the chemical vapor deposition (CVD) process, varying the boron quantity, the temperature and the flow rate. “Suddenly he found a material that looked completely different from a standard nanotube sample,” Terrones says. “It looked like a sponge with a fascinating texture.” It takes just 30 minutes to grow chunks as long as 4 inches and weighing 2 to 3 grams.
Hashim, however, had to shelve the research when Ajayan, his advisor, left RPI for Rice. When Hashim rejoined him as a graduate student in 2010, the massive Deepwater Horizon petroleum leak prompted them to redirect the research from batteries to environmental remediation. The work was supported by NSF, the Air Force Office of Scientific Research, the Army Research Laboratory and the Department of Energy’s Office of Science through ORNL’s Center for Nanophase Materials Science and the LCF. Other participating institutions include Spain’s Universidade de Vigo and Instituto de Microelectrónica de Madrid, the University of Illinois at Urbana-Champaign, the Air Force Research Laboratory and Arizona State University.
It should be relatively easy for manufacturers to modify bulk nanotube production processes to scale up nanosponge fabrication, Terrones says. Ultimately, how inexpensive the sponges become could be a function of the uses for them – and many are possible. They conduct electricity, so Sumpter sees applications in electronics and energy storage. Terrones says the material’s low density could make it a good scaffold for bone tissue growth. Impregnate the sponge with polymers and it could become a robust and light material for use in cars and planes. Hashim speculates the material even could be used to remove the salt from seawater.
For now, Hashim is continuing to explore ways to exploit nanotubes’ one-dimensional properties by engineering them into porous, three-dimensional solids. He’s focusing on enhancing the covalent bonding that makes them possible – and on finishing his doctoral studies. But, working with Ajayan and Rice, he’s also seeking ways to commercialize the discovery.