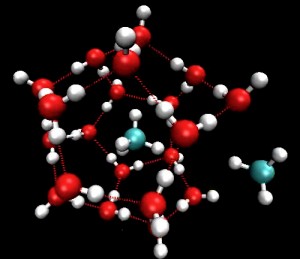
A still from an animation of methane, the blue and silver molecules, escaping from a methane hydrate, the red and silver molecules water molecules that form a cage around methane molecules. (Pacific Northwest National Laboratory.)
Hydrates are icy water-gas compounds abundant in ocean permafrost. They can hold numerous gases such as hydrogen, methane and nitrogen, a Pacific Northwest National Laboratory (PNNL) team’s supercomputer analysis of millions of molecular configurations reveals. Knowing the structure and molecular mechanisms of clathrates, the cage-like water molecules in compounds that host the gases, may help researchers find a feasible way to use hydrogen as an alternative fuel-storage system.
What’s more, the study suggests a mechanism governing how gas molecules hop between adjacent cages. A possible application for exploiting this feature: extracting methane from the hydrates and replacing it with carbon dioxide in a Davy Jones’ locker scenario to remove and safely store the potent greenhouse gas. The study took more than six months on Hopper, the Cray supercomputer at DOE’s National Energy Research Scientific Computing Center (NERSC).
Gas hydrates, long known to form at high pressures in deep ocean fossil fuel pipelines, were suspected as one culprit that complicated attempts to mend the Deepwater Horizon oil spill in 2010. At low pressure – Earth’s surface, say – methane hydrates are unstable and can be lit like an icy match, burning methane on the top as water drips at the bottom.
Researchers and entrepreneurs have begun to notice hydrates’ potential environmental applications.
“There is at least one large-scale effort by a company in Norway to take the methane out of these systems in the bottom of the ocean and try to pop carbon dioxide in there,” says PNNL Laboratory Fellow Sotiris Xantheas, who collaborated on the study with PNNL post-doctoral fellow Soohaeng Yoo Willow. “Take the gas out, use it as fuel for energy and fill the empty scaffold with CO2. You get double the benefit.”
Until Willow and Xantheas’ study, there had been scant information about how guest molecules like hydrogen interact inside clathrates’ hollow cages. Building on previous work, the team already had three-dimensional models of lattices made from cages of 20, 24 and 28 water molecules.
The model used for the study was made with 20 and 24 molecules. X-ray crystallography spelled out the oxygen atoms’ positions, and the team’s previous work suggested efficient ways to place hydrogen atoms in the lattice.
Imagine the clathrates’ cage of 20 water molecules as a soccer ball, Xantheas says. Because these are water molecules, 20 oxygen atoms on the surface means there must also be double that number – 40 – hydrogen atoms. “For the larger hollow cages comprised of 24 and 28 water molecules, there are millions of combinations you can do, and we figured out the most efficient ways and used numerous combinations in our simulations,” he says.
So far, so good, but this is just hydrogen in the lattice, not hydrogen placed inside it. “This is an important distinction,” Xantheas says. “One kind of hydrogen is very strongly bound to oxygen as part of the water molecule that forms the cage and it remains this way. Then there is the molecular form, H2, the guest molecule inside the cage: two hydrogen atoms connected that goes in and out of the cages. This can be used as a fuel.”
Xantheas and Willow built computer models packing this hydrogen gas inside the 3-D lattice. The simulations showed hydrogen escapes, reducing the cages’ storage capacity.
The explanation lies in the cages’ soccer ball shapes. Ones made of 20 water molecules have 12 pentagonal faces; the 24-water cage has 12 pentagons and two hexagons stitched together. The simulations showed molecular hydrogen mainly leaks through the hexagonal faces “because they are just too big,” Xantheas says. Other researchers tried using a larger molecule, such as tetrahydrofuran (THF), to keep hydrogen in, but that reduces storage capacity. “THF is a body by the door, blocking the exit, but it takes up space that ideally could go to hydrogen molecules.”
The PNNL team spurned THF. Since most clathrates contain methane the researchers wanted to swap for CO2, they instead added a methane molecule and found it kept hydrogen from escaping.
But they also were surprised to observe another low-energy way molecular hydrogen could hop between adjacent cages: a single hydrogen bond in the lattice breaks and allows one water molecule to rotate, opening a gate that closes as soon as the hydrogen passes through.
“The gate closes and the whole lattice is reformed and not destroyed,” Xantheas says. A computational experiment found the “water gate” also opens when the cage holds methane, suggesting it, too, can hop between adjacent clathrates without destroying the scaffold.
“We weren’t aware of this at all coming into the simulations,” he says. “This is a significant finding to see that this occurs in the natural world, and hopefully it puts other researchers on the path of future discoveries.” The PNNL team will follow that path by next trying to simulate exchanges between carbon dioxide and methane in the cages.
The discoveries are intriguing, but Xantheas says big, difficult challenges must be addressed before hydrogen becomes a viable fuel, including achieving system stability near ambient conditions while attaining higher storage capacity. “Even if you find a viable storage system and infrastructure, the economics will take over. But gas hydrates address two of the most environmentally important issues: alternative fuels and developing a safe system to sequester carbon dioxide. They are fascinating systems that show a lot of promise.”