Anyone who’s dealt with an uncooperative kid or combative employee is familiar with the concept of recalcitrance – stubbornness, disobedience and noncompliance.
It’s easy to see why scientists would apply the same term to lignocellulose. The woody material found in plant cell walls makes trees tough and stems stiff, but also locks up sugars, making it difficult to ferment them into ethanol fuel.
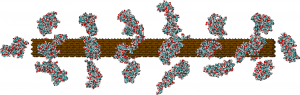
Atomic-detailed model of lignocellulose of softwoods. Based on experimental data on the structure of cellulose (brown) and lignin (cyan and red).
“Plants don’t want to be hydrolyzed and broken down into sugars. They develop these defense systems,” says Jeremy Smith, director of the Center for Molecular Biophysics (CMB), an Oak Ridge National Laboratory (ORNL)-University of Tennessee joint project. In other words, the plant materials that make up biomass for energy are recalcitrant.
Smith, who also is the first University of Tennessee (UT)-ORNL Governor’s Chair, and his fellow researchers are out to learn the atomic basis for this recalcitrance. With a 3.5 million processor-hour grant of computer time through DOE’s Innovative and Novel Computational Impact on Theory and Experiment (INCITE) program, Smith is building computational models of lignocellulose and testing how enzymes interact with it and each other. They’ll compare their models against neutron scattering experiments generated at ORNL’s Spallation Neutron Source (SNS).
The Oak Ridge CMB participates in the Department of Energy’s BioEnergy Science Center (BESC), an interdisciplinary coalition of experts from ORNL, UT and other universities, corporations and the National Renewable Energy Laboratory (NREL). The BESC mission is to achieve breakthroughs in biofuels from lignocellulosic biomass.
What they learn could help make cellulosic ethanol readily available and economically viable, helping the United States replace foreign oil with a renewable resource and curb greenhouse gas emissions. As it stands now, making ethanol from biomass like plant stalks or wood chips is difficult and expensive, requiring substantial energy and chemical inputs. Cellulosic ethanol production could generate less carbon dioxide and interfere less with food supplies, but it’s still easier and less expensive to make ethanol from corn and other crop-based feedstocks.
“We need to understand why (plant cell walls) are recalcitrant,” Smith says. “To do that you need to understand the structures of plant cell walls and what’s stopping them from being broken down by enzymes.”
Cellulose, the source of fermentable sugars in biomass, is locked inside some pretty tough stuff, as researched by CMB graduate student Benjamin Lindner and postdoctoral research fellow Loukas Petridis. Cellulose is contained in compact, partially crystalline fibrils that block enzymes. Polysaccharides and lignin cover the fibrils, presenting another barrier that enzymes must overcome. The lignin also may inhibit enzymes by attaching to their cellulose-binding components. In fact, removing lignin from biomass increases the cellulose-hydrolysis yield from 20 percent to 98 percent, the paper says.
Because of these barriers around lignocellulose, “You have to beat it up a bit before it will cooperate, and that’s expensive,” Smith adds. Computer simulation and neutron scattering experiments could provide clues on how to “kind of gently make (lignocellulose) cooperate in a way that’s economical, so we don’t have to heat it up to high temperatures or mix it with chemicals.”
Smith and his fellow researchers are setting up molecular dynamics simulations that will help them understand the physics of the chemical reactions involved in enzymatic hydrolysis of lignocellulose.
Once the lignocellulose model is complete, the research can turn to modeling how it interacts with enzymes that break lignocellulose down to sugars – and particularly how lignin keeps enzymes away. The enzymes of interest are cellulases, groups of proteins that are linked together but each serve a different function. Researchers want to know how the proteins work together, why they are attached and how this makes them efficient.
In Smith’s lab and with NREL, postdoctoral research associate Jiancong Xu is working on a molecular dynamics cellulosome model. Meanwhile, Moumita Saharay, another postdoc in Smith’s lab, is combining molecular dynamics and quantum mechanics to model how enzymes break and reform chemical bonds to break down cellulose.
The simulations explicitly recognize each atom in the molecules they portray, Smith says. Because the simulations can track a million or more atoms at once, “We need big supercomputers to perform these calculations,” he adds.
At Oak Ridge, Smith and the rest of his group use Jaguar, a Cray XT4 with an aggregate system performance of 263 teraflops. Jaguar ranked as the world’s fifth most powerful computer in the June 2008 TOP500 list. Quad-core processors have boosted Jaguar’s power in recent years, but Smith and his fellow researchers want more.
“We are so hungry for computing power because we can never get enough. It’s really what limits the usefulness of these simulations,” he says. “A million atoms seems like a lot but it’s only a tiny sliver of biomass. We’d also like to simulate it for milliseconds or a second. (Now) we can simulate it only a microsecond.”
Smith’s group plans to integrate their simulations with results from another major facility at Oak Ridge: The Spallation Neutron Source, which generates the most intense pulsed neutron beams in the world. How neutrons scatter when they strike protein molecules provides clues about how atoms are arranged in those molecules.
“The simulations are a way of interpreting the neutron experiments, so the neutron experiments and simulations will be performed simultaneously,” Smith says.
Smith and his fellow researchers are only beginning to refine their computational models. “You can be sure that for the next year or two we’ll get everything wrong,” he joked, as models are tweaked and refined. “We’ll do simulations that are not quite the real thing, but then eventually we’ll get a model that seems to agree with everything,” creating a powerful tool to help design new plants or enzymes that make lignocellulose more amenable to ethanol production.
A longer version of this article originally appeared in the print DEIXIS.